Please click here to access the main AHDB website and other sectors.
- Home
- Knowledge library
- Diseases
Diseases
The traditional model of disease causation or the ‘epidemiological triad’ requires the interaction of three things; a susceptible host, a virulent pathogen and an environment favourable for disease development.
Growers need to eliminate just one of these causal agents to manage a disease. Diseases can be particularly difficult to control in glasshouses as the conditions in which crops thrive are the same as many diseases. Managing the glasshouse environment to favour crop growth but minimise the risk of disease infection is a balancing act for growers. Plants will be more susceptible to disease when stressed so optimising growing conditions (air, water, light, nutrients, heat, CO2) is key.
Some main diseases with carry-over issues for growers that AHDB have researched include:
- Rhizobium (previously Agrobacterium, root mat), is a member of a small group of unusual plant diseases (neoplastic diseases) in which the infecting bacterium, usually a species of Rhizobium, causes new plant tissues to grow.
- Botrytis (grey mould), a nectrophic fungus that affects many plant species, especially common in humid glasshouse conditions affecting weak plants and attacking through wound sites.
- Oomycete fungi;
- Phytophthora, causes root, stem, crown and foliar rots and blights. Common in the glasshouse due to warm temperature, humidity and water for spread. It has multiple dissemination pathways with different spore types.
- Pythium, crown and root rot, a very common water mould that can severly damage roots resulting in stunted growth and plant death. Commonly introduced in replanted crops such as cucumber. Favoured by poorly-draining media and is most problematic in stressed crops.
- Fusarium, can be found on stems or nodes or wound sites. It thrives in high humidity and is spread through airborne inoculum. Fusarium wilt enters through the roots and interferes with water conduction. Often exhibited under the stress of full fruit production.
- Powdery mildew, an obligate parasite with different species affecting a number of crops causing white fungal growth mainly on the upper surface of foliage.
- Bacterial wilt and canker, causes wilting and plant collapse in tomatoes, usually introduced on infected seed or plants.
- Verticilium wilt, causes wilting and staining of vascular tissue, spores spread from stems
- Myco (Mycosphaerella melonis), gummy stem blight, is a fungal pathogen of cucumber, it causes stem and leaf infection and fruit end rot. It can disperse by conidia and ascospores.
There are a number of other crop specific disease issues, for example Sclerotinia and Rhizoctonia that have may have been investigated for in-season controls (see AHDB Horticulture research library for research reports) but not specifically in terms of preventing carry-over.
Growers are recommended to consult their AHDB crop walkers' guides as an aid to identifying unfamiliar symptoms. Find these listed on the crop index pages, or search for them via the Knowledge Library.
Rhizobium (Agrobacterium, root mat /crazy roots)
Root mat (RM) was first noted in soil and straw-bed cucumbers in the UK in the 1970’s but did not become a problem again until 1993 in a rockwool crop, in tomatoes the disease was first observed in the UK in 1999. PC 149 ‘Cucumber and tomato: investigation of the cause, epidemiology and control of root proliferation ('rootmat') in hydroponic crops’ was initiated in 1997 and the final report three years later concluded:
- Eight disinfectants reduced growth of Agrobacterium in a broth suspension test. Three were ineffective at the rates tested.
- Five disinfectants were effective when tested against Agrobacterium dried on concrete.
- None of three water treatments tested were both safe to growth of young cucumber plants and effective in controlling Agrobacterium.
- Spray application, after high-pressure washing with water, was effective in disinfecting irrigation drip pegs naturally contaminated with Agrobacterium.
- Steaming rockwool slabs for 5 hours eliminated Agrobacterium bv 1 but did not destroy the rhizogenic Ri plasmid. Non-rhizogenic Agrobacterium bv 1 added to the steamed slabs was able to acquire the Ri plasmid.
- Two out of 13 potential biocontrol organisms significantly delayed the occurrence of root mat symptoms in an inoculated cucumber crop. On re-testing these two organisms (Pseudomonas sp. and Agrobacterium radiobacter K84) the following season, the latter organism again suppressed the disease, but the Pseudomonas isolate was not effective.

PC 241 ‘Protected hydroponic tomato: Investigating the potential for various novel non-chemical techniques for the suppression or control of root-mat disease’, was initiated in 2006. The project suffered with lack of symptom expression in trials on a research site and commercially. The grower action points were:
- Liaise with propagators regarding hygiene measures and possible applications of biological control products pre-delivery to nursery.
- Consider the use of biological products during the first two months after the crop is planted.
- Maintain a normal clean-up routine between crops, avoid high hygiene scenarios unless other pathogen outbreaks dictate decision.
- Monitor crops and feed information back to members of the project team if root-mat problems increase on your nursery.
The two industry representatives also made some useful comments:
‘When dealing with this disease in the production nursery it would appear to be best to do no more than a normal clean-up. Trying to do more than this may increase the level of the problem rather than reduce it by removing organisms antagonistic to RM. However, clean-up action in propagation to remove any trace of the plasmid will reduce early infection and does seem to be more effective in reducing the level of infection in the mature crop. This action in the propagation area should include the drains where plasmid may be found and any trays used for delivery as these may carry infection back from infected nurseries into the propagation nurseries.’ Derek Hargreaves.
‘Experience with this problem suggests that ‘dirtier is cleaner’ in any attempt to get rid of this problem we need to understand more about microbial competition. Areas diligently cleaned with several biocides seem no more cleaned up of the plasmid than moderately cleaned areas. Growing in soil (with a high biological diversity and thus competition for the causal agent) appears to be the best option resulting in reduction of symptoms. This may not be practical of course and new research is currently looking at biological solutions for use in inert media. It also appears that plant stress plays an important role in symptom expression with increased stress resulting in more severe symptoms.’ Dr. Phil Morley.
In 2016 a new piece of work was undertaken, PE 029 ‘Protected tomato: Evaluation of biological treatments, biocides and an improved diagnostic for control of root mat disease’. The aim of the project was to identify biological treatments and biocides that reliably control or suppress root mat disease by prevention of infection and transformation of protected tomato by bacteria carrying the root initiation plasmid (pRi) and to develop a rapid molecular test for early detection of infected plants. Once a plant is infected with the plasmid, there are no known treatments which will prevent symptom development. Consequently, the current focus for control of disease is to prevent infection. There is good reason to believe biological treatments could reduce tomato root mat by influencing the population of rhizogenic bacteria around tomato roots. A number of mainly biological products are being tested in primary screens. The first year of results have found a reduction in root mat symptoms with several biological products tested, further information can be found in the project report PE 029.
There have been some recent industry funded trials in Holland that suggest the bacterium which causes root mat develops less easily on organic substrates than on mineral substrates. PC 149 (2000) included a small inoculated trial to investigate the effect of growing medium on root mat in tomato. One month after sowing and immediately before planting, slabs were inoculated with rhizogenic R. radiobacter. At 12 weeks after inoculation, root mat severity was significantly less in coir slabs (0.7 on 0-4 index) than in rockwool (1.9) or peat slabs (1.6). PE 029 found differences in observation trials between organic media with different chip:pith ratios on a commercial site. When slabs were tested they were not found to differ significantly in key substrate properties and it was concluded that some other aspects on site are affecting root mat incidence (e.g. scion variety, inoculum load of surroundings) or that the substrates do differ in some way not examined.
Botrytis cinerea (grey mould)

In 1995 PC 098 ‘Long season tomatoes: control of stem botrytis by treating young lesions’ investigated the application of products to stem lesions to prevent Botrytis spread. An extension of the work PC 098a ‘Long-season protected tomatoes: control of stem Botrytis’ found removal of old brown trusses to be important in reducing spread and a clean removal of the trusses with no butt was essential.
The action points from PC 098a were:
- Minimise the risk of Botrytis by maintaining effective hygiene standards in the crop.
- During periods when the weather conditions are conducive to infection make every effort to reduce the relative humidity by applying ventilation and pipe heat.
- Old fruit trusses appear to be a major source of stem Botrytis. Consider routine removal of old trusses as they brown to reduce the risk of stem Botrytis.
- Whatever method is used to remove trusses ensure that no stub is left attached to the stem. The presence of a stub considerably increases the risk of Botrytis developing at the site of truss removal.
- Inspect crops regularly for Botrytis stem and foliar lesions.
- Consider submitting a random selection of leaves or stem pieces affected by Botrytis infection to your plant pathology laboratory to determine the sensitivity of the fungus to a range of fungicides.
- When disease pressure increases or after trimming operations in the crop consider applying a protective fungicide programme according to normal commercial practice.
Work in PC 174 ‘Tomatoes: development of biocontrol as a component of an integrated, sustainable strategy for the control of grey mould (Botrytis cinerea)’, showed several biocontrol products and micro-organisms, gave significant reductions of tomato stem Botrytis in laboratory tests and glasshouse trials. Old fruit trusses were confirmed as an important infection route leading to stem Botrytis.
AHDB Horticulture project PC 301 ‘Targeting of humidity control, through the use of stem temperature measurements, to reduce stem Botrytis and save energy in tomato production’ concluded that stem temperature monitoring and associated humidity control are clearly a significant step forward compared with reliance on control driven by general air humidity sensing.
AHDB Factsheet 23/11 ‘Grey mould (Botrytis cinerea) of tomato’ was produced after project work in PC 301. It summarised sources of infection and spread.
The fungus produces survival structures called sclerotia that can live up to about 20 years in dried plant material so a thorough clean-up of the glasshouse environment is critical.
Oomycete fungi

Oomycetes are fungus-like pathogens that cause a range of symptoms in many horticultural crops. Also known as the ‘water moulds’ and water is an important means of spread (see Water). In the protected sector Pythium and Phytophthora species are particularly problematic due to their ability to produce highly resistant oospores that can easily overwinter or survive as zoospores in a water source.
In the series of AHDB Horticulture work project; PC 281 ‘Protected tomato: Monitoring rhizosphere micro-organisms to improve understanding and management of root diseases’, PC 281a ‘Tomato: application of next generation diagnostics for improved detection and understanding of root diseases’, PC 281b ‘Tomato: Micro-organisms in the irrigation water of hydroponic crops grown in closed systems’ a useful general conclusion is that the use of grafted tomato plants appears to reduce the risk of severe root disease. Several plant pathogens were detected in the roots of Beaufort, Maxifort and Emperador and yet the crops grew and yielded well, with no yellowing, wilting or stunted growth.
AHDB has engaged a programme of work on Oomycetes and there are web pages on the AHDB website with management guidance. AHDB has funded several projects from 2014; CP 126 ‘A desk-study to review global knowledge on best practice for Oomycete root-rot detection and control’, CP 128 ‘Development and delivery of knowledge transfer activities on current best practice for Oomycete root rot detection and control’, CP 136 ‘Diagnostic: Development of Oomycete LFDs’ and CP 157 ‘Aerial oomycetes: A review of management and control options available for the UK horticulture industry‘
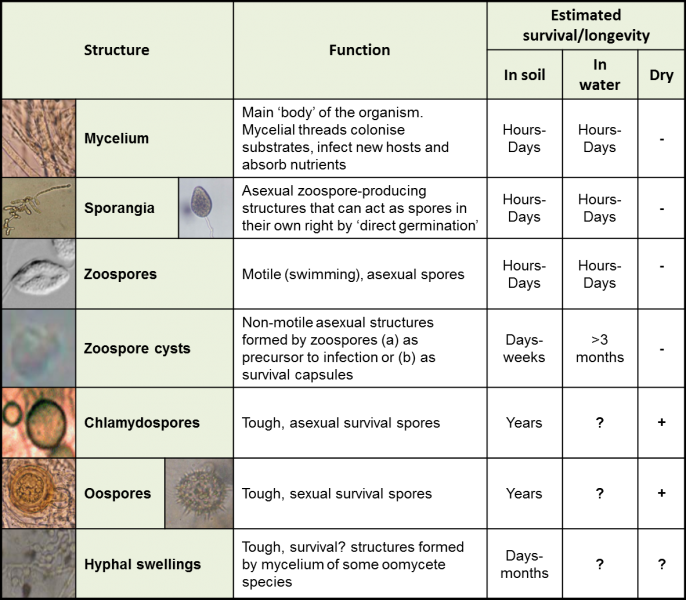
Typical oomycete spore types and growth structures, their functions and estimated survival in various environments.
Fusarium
Various Fusarium species cause stem, fruit and root rots and wilts in protected crops. PC 281 ‘Protected tomato: Monitoring rhizosphere micro-organisms to improve understanding and management of root diseases’, found abundant levels of Fusarium in roots of all types of growing media with monitoring in successive years showing these pathogens persisted season to season despite water treatment. A summary of several projects investigating Fusarium in peppers can be found in AHDB Factsheet 05/15 ‘Pepper fruit rots’ where the need to ensure good crop hygiene and end of season clean-up is stressed.
Fusarium lives on dead plant tissue and in soil, and overwinters as thick-walled, resistant spores. Thorough end of season clean-up and on-going removal of any affected plants and fallen fruit is important in control of this disease.

Powdery Mildew
Powdery mildews affect many glasshouse crops. Infection, as small whitish powdery patches, is usually first seen on the upper surface of leaves but can also begin development on the lower side, stem and trusses. Fungal pathogens causing powdery mildew have narrow host ranges so are quite specific but sometimes weeds can be hosts and sources of infection.
Powdery mildews are can be controlled by choice of resistant varieties and spray programmes (although powdery mildew pathogens have a high potential for fungicide resistance). They are mainly obligate pathogens so require living host plant tissue to survive overwinter. If all crop debris is cleared between crops fungal pathogens causing powdery mildews should be prevented from overwintering.
There is little specific AHDB work on powdery mildew in the protected edibles sector. There was research in 1991, PC 026 ‘Tomato: chemical, cultural and biological control of powdery mildew’. In 1994 an extension, PC 026a ‘Fungicide sensitivity testing and comparison of fungicides for tomato powdery mildew’ was carried out but product approvals must be checked due to the age of the work. There is a body of work for strawberries with a Factsheet 29/16 ‘Control of powdery mildew under protection’ summarising previous research. There are similar publications for cut flowers, 11/03, hardy nursery stock, 17/10 and Brassicas (brassica diseases guide).
Powdery mildew is particularly problematic in cucurbits so has been included in work to develop on-site tests to monitor glasshouse bio-aerosols, CP 137 ‘Cucumber: Development and testing of a lateral flow device for both gummy stem blight and powdery mildew in bio-aerosols during cucurbit production’.
Powdery mildew was also a focus disease in SCEPTREplus.
Bacterial wilt and canker

Bacteria are dependent on outside agents (often staff) to move them from plant to plant as most do not produce spores which is a common method to spread fungal diseases.
Bacterial canker is the most serious bacterial disease of tomatoes worldwide. It nearly always causes surprise by its sudden appearance partway through the season and usually on a nursery with no recent history of the disease. This is generally due to a long period of latent, systemic infection in young plants. Infected seed or young plants are the most common source of infection.
Glasshouses and any other areas that may have come into contact with the bacterium should be thoroughly sanitised before a new tomato crop is planted. This includes trolleys, irrigation equipment, support wires, bobbins, flooring, concrete pathways and the glasshouse structure. Recent experience in the UK where infection has re-emerged in the same location in one glasshouse in successive years strongly indicates that Cmm can persist from one season to the next on bobbins and drippers, so these should be thoroughly disinfected or renewed after an outbreak.
An AHDB Factsheet 01/10 ‘Bacterial wilt and canker of tomato (Clavibacter michiganensis subsp. michiganensis)’ on this disease was produced in 2010.
Clean seed and good hygiene particularly early in cropping, is critical as once established bacterial diseases are difficult to eradicate and crops can withstand infection better in later stages.
Verticilium wilt
A virulent strain of Verticilium wilt hit tomato crops in 2001. An AHDB Horticulture project PC 186 ‘Tomato: an assessment of current problems and future risks of Verticillium wilt in hydroponic and soil-grown crops’ was undertaken and outlined control measures with useful details on end of season clean-up in AHDB Factsheet 15/01 ‘Act now to control Verticillium wilt of tomatoes’.
In summary it advised:
- Two days before the crop is to be removed, spray the stem bunches with a compound mixture in order to prevent spread from the basal lesions or fumigate the crop.
- Remove the crop and as much plant debris as possible preferably folding it into the polythene floor covering to take out of the house. Do not re-use the slabs for any other glasshouse crop or strawberries.
- Power-wash all surfaces with water and allow the greenhouse to dry.
- Spray the whole of the inside of the greenhouse, including the irrigation equipment, with disinfectant. Soak all pegs in this disinfectant for at least 1 hour.
- Spray all equipment including trimming trolleys with disinfectant.
- Spray all concrete surfaces both inside and out with disinfectant giving particular attention to areas where debris has stood during the season.
- Thoroughly wash all irrigation tanks that may have been contaminated with plant debris or sciarid flies and disinfect thoroughly washing out 24 hours after treatment.
- Kill all sciarid flies if any have survived the clean-up treatments.
- Re-sheet the greenhouse with polythene and put the slabs in place. Re-spray the house with disinfectant when this job has been completed and then close the house for at least 24 hours with a minimum temperature of 15°C.
- Air the house ready for the arrival of new plants.

Myco - Mycosphaerella melonis (gummy stem blight)

AHDB Horticulture has funded a series of work on gummy stem blight which is problematic for cucumber growers:
- PE 001 ‘Cucumber: Improving Control of Gummy Stem Blight caused by Mycosphaerella melonis (Didymella bryoniae)’,
- PE 001a ‘Cucumber : Improving Control of Gummy Stem Blight caused by Mycosphaerella melonis (Didymella bryoniae)’.
Good hygiene in the crop and thorough end of season clean-up are important parts of the management strategy. An AHDB Factsheet 07/15 ‘Cucumber: Epidemiology and Control of Mycosphaerella’ with further information is available.
The action points from the Factsheet are:
- Remove all plant debris from the glasshouse and the surrounding area as soon as possible at the end of cropping.
- Where plant material is left on site temporarily, e.g. in skips, then cover it to prevent release of Mycosphaerella spores. If material is retained on site for composting, it must be covered to reduce the risk of spore dispersal.
- Thoroughly clean the glasshouse and all equipment, ensuring all plant debris including tendrils are removed from crop wires. Use a detergent wash to remove organic debris on surfaces and then disinfect using an appropriate disinfectant.
- Lay the polythene floor coverings carefully using two teams to prevent contamination of the new surface. Pay particular attention to the side walls, the junction with the roadway and the area around the concrete dollies supporting the structure to ensure you cover all soil. Check all the gutters and fix any drips or leaks.
- Use healthy plant material from a reputable and trusted supplier.
- Although there are no Mycosphaerella-resistant cultivars, susceptibility varies so choose a cultivar with minimum susceptibility, especially to internal fruit rot; though don’t compromise on mildew resistance in the process.
- Take particular care with the new plants to avoid secondary contamination. Do not place on dirty surfaces or in areas where there is a risk of wind-blown dust, e.g. doorways.
Work in these projects has led to a cross sector project developing an on-site lateral flow device, AHDB Horticulture project CP 137 ‘Cucumber: Development and testing of a lateral flow device for both gummy stem blight and powdery mildew in bio-aerosols during cucurbit production’ see Future advances.
Images courtesy of:
Rhizobium sp. - AHDB Crop Walker Guide (Derek Hargreaves)
Oomycete fungi. CP 126 (Tim Pettitt)
Bacterial wilt and canker on tomato fruit - AHDB Crop Walker Guide (Derek Hargreaves)
Fusarium spp. - AHDB Crop Walker Guide (Derek Hargreaves)
Mycosphaerella melonis (gummy stem blight) on cucumber fruit - AHDB Crop Walker Guide (Derek Hargreaves)
Sectors:


