Please click here to access the main AHDB website and other sectors.
- Home
- Knowledge library
- Water-powered dilutors: the calibration process
Water-powered dilutors: the calibration process
Calibrating your water-powered dilutor regularly is essential. It should be done at least once a year, and preferably every six months to verify that the correct levels of soluble fertilisers (and other substances) are being applied to crops through the unit. Learn more about the detail of the calibration process.
This information was last updated in 2018.
Go back to the main calibrating a water-powered proportion dilutor page
Equipment
You will need the items listed to calibrate the dilutor (See figure 1):
- Scales reading in one gram intervals to 2,000g (2kg), with a zeroed reading facility
- A small measuring cylinder capable of measuring up to 20ml in 5ml intervals
- A range of bottles or beakers with capacities of 500mls
- An electrical conductivity meter which has temperature compensation built into it
It is important to have calibration solutions for the electrical conductivity meter and to calibrate the meter at least every four to six months. Generally electrical conductivity meters are far more reliable and robust than pH meters.
 Substrate Associates Ltd
Substrate Associates Ltd
Figure 1. Essential items of equipment that are required to undertake the calibration procedure.
Calibration procedure
Make up a fertiliser stock solution using any commonly available water soluble fertiliser, or use a readily available fertiliser stock solution which would normally be applied to the crops grown.
For example, take a compound water soluble fertiliser such as 20-10-20 (N:P:K) and make up a stock solution containing 100g/l.
- Dissolving the crystalline fertiliser in the water will reduce the temperature of the solution and it should therefore be left for a short while to reach ambient temperature.
- If possible, use warm water (say 15–20°C) to help dissolve the crystals, and stir the solution continuously to ensure the fertiliser is fully dissolved.
Measuring out
When measuring out small amounts of liquid it is often easier and more accurate to use scales rather than go by the graduation marks on the container. As one millimetre of water equates to one gram in weight, this makes the process straightforward.
- Zero the scales with the container on them and weigh out the corresponding amount of solution.
- Weigh out 10g of the water soluble fertiliser stock solution into a small container using the small measuring cylinder. (The type of plastic measuring thimble supplied with cough medicines is accurate enough for this).
- Pour the measured amount of stock solution into a clean, dry beaker or bottle capable of holding 500mls plus of solution, already placed on a set of scales which have been zeroed, so that the recorded weight is 10g.
- Now pour in water from the nursery/farm supply until the reading on the scales is 500g. At this point the beaker contains a diluted solution of 1 in 50 (2%).
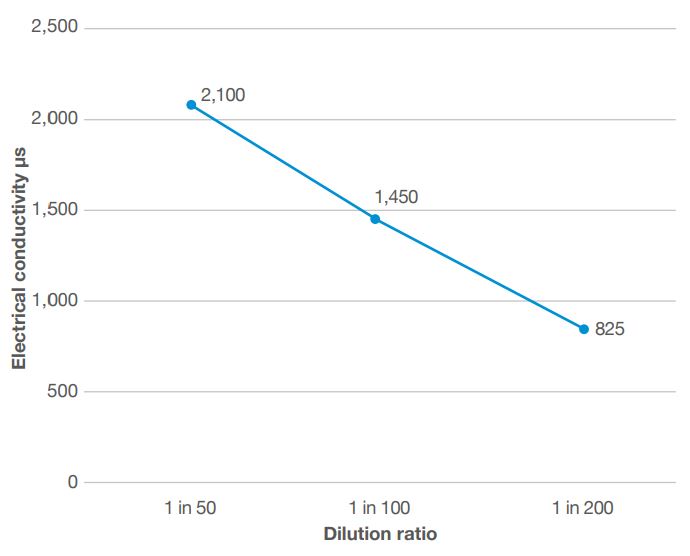
Measure the electrical conductivity (EC)* of the diluted stock solution just created. This will be the highest value recorded and will probably be around 2,100µS/cm2 (microsiemens) or 2.1mS/cm2 (millisiemens), depending upon the EC of the water used during the calibration procedure.
- Record the value obtained.
- Take a fresh bottle or beaker and place it on the scales and again zero the reading. Weigh into this bottle 50ml of the previously diluted (2%) solution. This should read 50g on the balance.
- Add to this a further 50mls of the nursery/farm water supply such that the balance now reads 100g.
Dilution rates
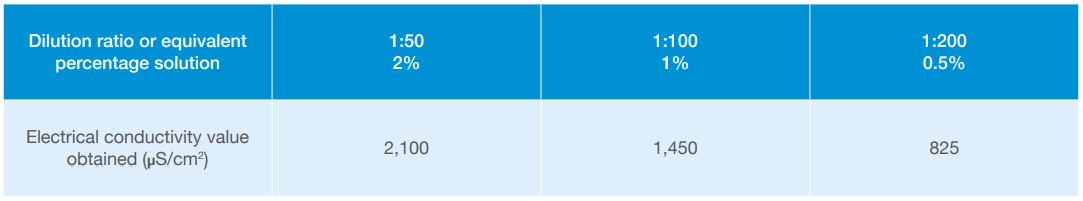
Figure 2. EC values obtained from the calibration procedure
The solution created has now been diluted to 1 in 100 (1%). Measure the EC of this solution; the value will probably be around 1,400–1,500µS/cm2 or 1.4–1.5mS/cm2
- Record the value.
- Finally, in a clean bottle or beaker, again zeroed on the scales, weigh out 50g of the previously diluted solution (1%) and add to it a further 50mls of the nursery/farm water supply.
This solution will now be a dilution of 1 in 200 (0.5%). Measure the EC of this solution, which will be in a range of 700–900µS/cm2 or 0.7–0.9mS/cm2, and record the value.
The values can be recorded as demonstrated in the table below, noting the EC level found against each of the sequential dilution ratios.
Calibration curve
Figure3. Example of a calibration curve which can be used to assess the performance of on-site proportion dilutors via the predicted EC levels.
The calibration curve created from the results of the calibration procedure can be used to test the output of the dilutor at a specific dilution setting.
- Take the stock solution used to produce the calibration curve and run it through the proportional dilutor to be assessed.
- Collect the output solution and measure the EC of the solution and compare it to the calibration curve (Figure 3).
For example, at a setting of 1 in 100 (1%) on the proportional dilutor, an EC reading of 1,300µS/cm2 may be obtained, where the calibration curve predicted a value of 1,450 µS/cm2.
To compensate for this, the dilution setting on the dilutor can be adjusted, possibly to 1 in 80 (1.25%). To check this:
- take a second sample
- measure the EC to see if the reading is nearer to the value required.
- this uses the setting on the dilutor as an indicator and the actual accuracy is measured by the use of the EC value.
Experience has shown this task to be essential; a regular check is required to verify that the correct levels of soluble fertilisers (and other substances) are being applied to crops through the unit.
Note that all the values obtained include the EC of the nursery/farm irrigation water from the source used. Water in the U.K. can have EC values varying between 50µS/ cm2 and 1,200µS/cm2, depending upon source and geographic location.
The water used in the calibration procedure recorded in the table [fig 2, p.3 of factsheet] had an EC of 100µS/cm2; all the values shown in the table could only be used for another water source if they were first reduced by 100 and then had the new irrigation water EC value added onto them.
* Electrical conductance (EC) is a measure of the ease by which an electrical current passes through a solution or substance; one millisiemen (mS) equates to one thousand microsiemens (µS).
Useful links
Read: Water-powered dilutors - unit maintenance
Download the factsheet version of this information
Acknowledgements
The guidance is based on the content of Bulrush Horticulture Grower Notes GN10. Thanks also go to Russell Woodcock and Gary Woodruffe, Bordon Hill Nurseries, for their time and input into the production of this information and the video dealing with the calibration of water-powered proportional dilutors.